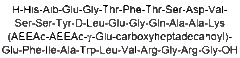The semaglutide impurity des-14-Leu-SMT is a structurally related impurity that may be produced during the synthesis or degradation of semaglutide (a GLP-1 receptor agonist used to treat type 2 diabetes and obesity). This impurity lacks the leucine residue at position 14 (des-14-Leu), which may cause changes in the molecular structure and thus affect the purity and biological activity of the drug. In drug quality control, this impurity needs to be strictly monitored to ensure the safety and efficacy of semaglutide.
Product Overview
|
Basic Description |
Structure and function: |
|
Three-letter sequence |
H-His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Glu-Gly-Gln-Ala-Lys-Glu-Phe-Ile-Ala-Trp-Leu-Val-Lys-Gly-Asp-Val-Arg-Arg-Arg-Arg-Arg-Arg-Gly-CONH2 |
|
Single letter sequence |
HHAEGTFTSDVSSYEGQAKKEFIAWLVKGDVRRAARRRRG-NH2 |
|
Molecular formula |
C183H284N51O56S |
|
Molecular weight |
4034.16 g/mol |
Product Attributes
|
purity |
>98% |
|
form |
Lyophilized powder |
|
Storage conditions |
Store at -20°C/-80°C away from light |